Emeset Tablets (Generic Zofran) - Active Ingredient And Chemical structure
The active ingredient contained in Emeset Tablets is Ondansetron HCl. Ondansetron HCl dihydrate is a white to off-white powder that is soluble in water and normal saline. The structure is shown below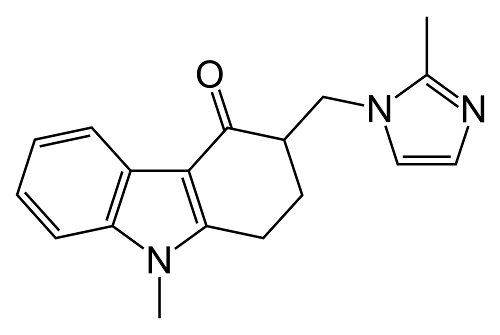
Generic Zofran (Emeset Tablets) - Uses
Generic Zofran in the form of Emeset Tablets is used for the following Indications:
1.Prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy, including cisplatin = 50 mg/m2.
2. Prevention of nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy.
3. Prevention of nausea and vomiting associated with radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen, or daily fractions to the abdomen.
4. Prevention of postoperative nausea and/or vomiting. As with other medications used to prevent vomiting and nausea, routine prophylaxis is not recommended for patients in whom there is little expectation that nausea and/or vomiting will occur postoperatively. In patients where nausea and/or vomiting must be avoided postoperatively, ondansetron tablets and syrup is recommended even when the incidence of postoperative nausea and/or vomiting is low.
Generic Forms and Brand names of Zofran
Emeset Tablets manufactured by Cipla Ltd., India is an effective treatment for the prevention of nausea and vomiting that may be caused by surgery or by medicine to treat cancer (chemotherapy or radiation). Zofran which has the active ingredient Ondansetron HCl is also sold as Zuplenz, Emetron, Emodan, Ondemet, Setronax, Ondavell and under various other brand names.
Emeset Tablets Preparations
Emeset is available as tablets of 4 mg and 8 mg . Each film coated tablet contains Ondansetron hydrochloride dihydrate Equivalent to Ondansetron 4 mg and 8 mg respectively.
Emeset Tablets - Storage Requirements
Emeset Tablets are to be stored at room temperature (15°C to 30°C). Store away from heat, moisture, and light.
Emeset Tablets - Dosage
Prevention of Nausea and Vomiting Associated With Highly Emetogenic Cancer Chemotherapy: The recommended adult oral dosage of Emeset Tablets is 24 mg given as three 8-mg tablets administered 30 minutes before the start of single-day highly emetogenic chemotherapy, including cisplatin = 50 mg/m2.
Prevention of Nausea and Vomiting Associated With Moderately Emetogenic Cancer Chemotherapy: The recommended adult oral dosage is one 8 mg Emeset Tablet given twice a day. The first dose should be administered 30 minutes before the start of emetogenic chemotherapy, with a subsequent dose 8 hours after the first dose. One 8 mg Emeset Tablet should be administered twice a day (every 12 hours) for 1 to 2 days after completion of chemotherapy. For pediatric patients 12 years of age and older, the dosage is the same as for adults. For pediatric patients 4 through 11 years of age, the dosage is one 4-mg Emeset Tablet given 3 times a day. The first dose should be administered 30 minutes before the start of emetogenic chemotherapy, with subsequent doses 4 and 8 hours after the first dose. One 4 mg Emeset Tablet should be administered 3 times a day (every 8 hours) for 1 to 2 days after completion of chemotherapy.
Prevention of Nausea and Vomiting Associated With Radiotherapy, Either Total Body Irradiation, or Single High-Dose Fraction or Daily Fractions to the Abdomen: The recommended oral dosage for adults is one 8 mg Emeset Tablet given 3 times a day.
For total body irradiation, one 8-mg Emeset Tablet should be administered 1 to 2 hours before each fraction of radiotherapy administered each day.
For single high-dose fraction radiotherapy to the abdomen, one 8-mg Emeset Tablet should be administered 1 to 2 hours before radiotherapy, with subsequent doses every 8 hours after the first dose for 1 to 2 days after completion of radiotherapy.
For daily fractionated radiotherapy to the abdomen, one 8-mg Emeset Tablet should be administered 1 to 2 hours before radiotherapy, with subsequent doses every 8 hours after the first dose for each day radiotherapy is given.
Postoperative Nausea and Vomiting: The recommended dosage is 16 mg given as two 8 mg Emeset Tablets 1 hour before induction of anesthesia.
Emeset Tablets - Contraindications
The concomitant use of apomorphine with ondansetron is contraindicated based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron. Emeset Tablets are contraindicated for patients known to have hypersensitivity to Ondansetron HCl or any inactive ingredient present in the medication.
Emeset Tablets - Overdosage
There is no specific antidote for ondansetron overdose. Patients should be managed with appropriate supportive therapy. Individual intravenous doses as large as 150 mg and total daily intravenous doses as large as 252 mg have been inadvertently administered without significant adverse events. These doses are more than 10 times the recommended daily dose.
Side Effects Of Emeset Tablets (Generic Zofran)
The most common side effects of are Headache, Diarrhea, Malaise/fatigue,
Constipation and Dizziness.